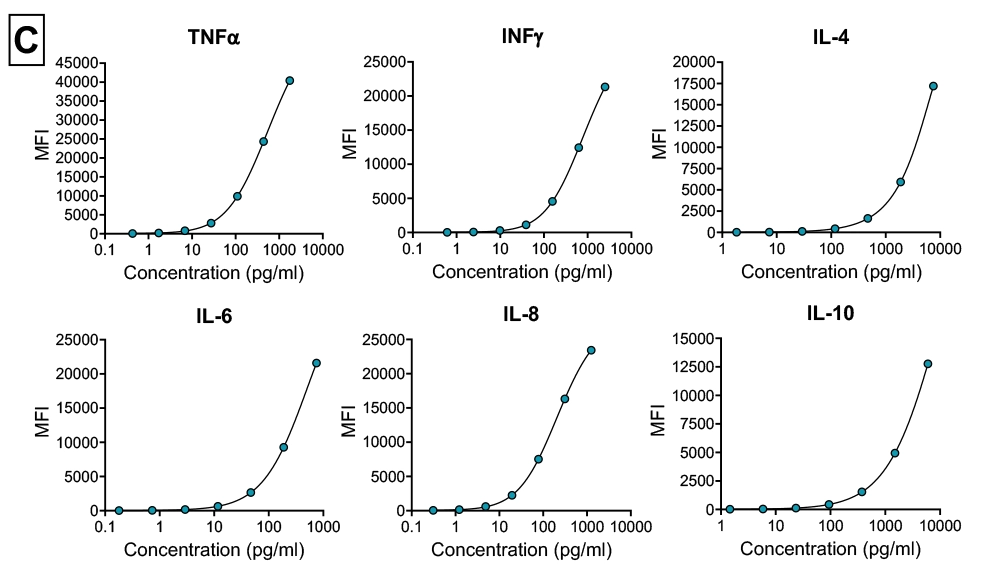
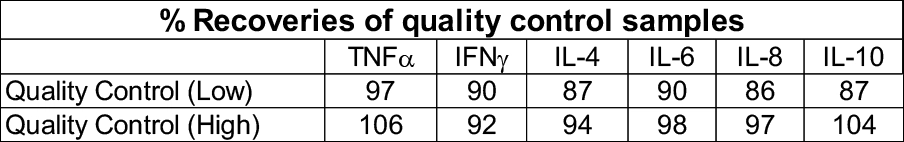
Cytokine storm assay (Immunogenicity)
In vitro assay using PBMCs isolated from healthy donor whole blood. Suitable as a predictive model for immunogenicity risk assessment (Cytokine storm). Assay utilises Luminex multiplexing magnetic bead-based assay detection.
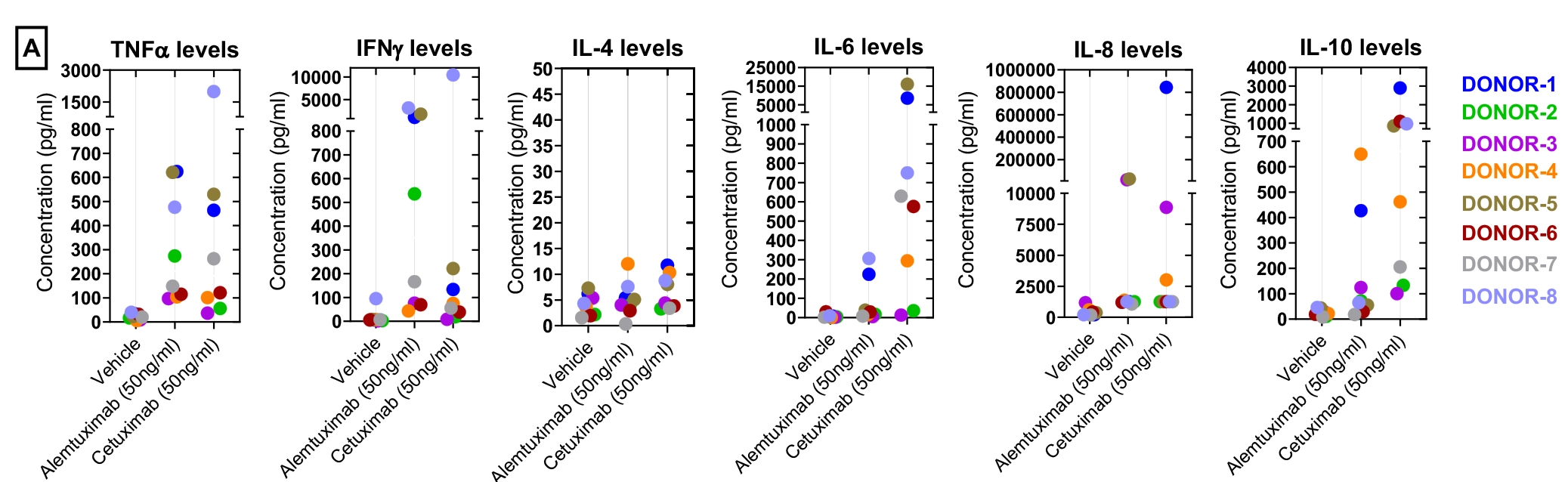
Figure-A: Concentration of each Cytokine analyte (pg/mL) in PBMC supernatant from eight-donors. Data was plotted as y-axis (cytokine levels in pg/ml) versus x-axis (test condition). Data points within each donor were represented with one colour code. Each point represents average data from duplicate wells.
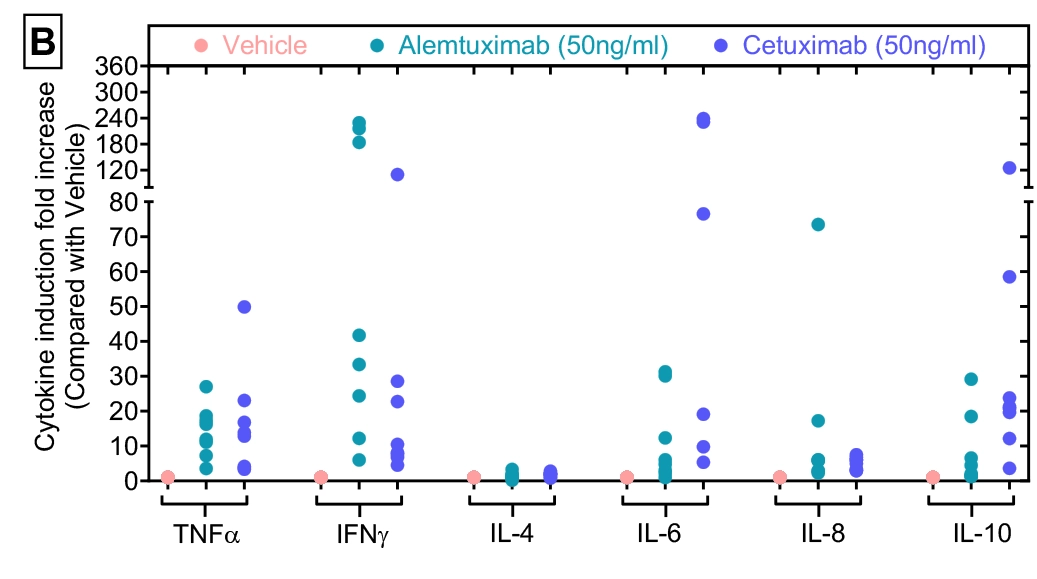
Figure-B: Cytokine induction fold increase against eight donors. Cytokine levels (pg/ml) data shown in Figures-A was re-analysed to measure Cytokine induction fold increase in the presence of agents over vehicle control. Data was plotted as y-axis (Cytokine induction fold increase over Vehicle) versus x-axis (test condition within each cytokine).
The response exhibited by Alemtuzumab and Cetuximab in this assay agrees with clinical immunogenicity findings in the literature. In vitro Cytokine storm assays developed at BioMedha are robust tools for implementing and assessing immunogenicity risk at an early stage. Understanding and managing immunogenicity at the earliest possible stage is important to improve safety and efficacy of therapeutic molecules in drug development.