Residual host cell DNA quantification
Host cell lines like CHO, E. coli, and HEK293 are used to produce therapeutic proteins. The presence of residual host cell DNA in biopharmaceutical products carried into the body may lead to an increased oncogenicity and/or immunogenicity risk. To mitigate the risk, biopharmaceutical samples were analysed for host cell line residual DNA quantification by qPCR.
Residual CHO cell line DNA Quantification
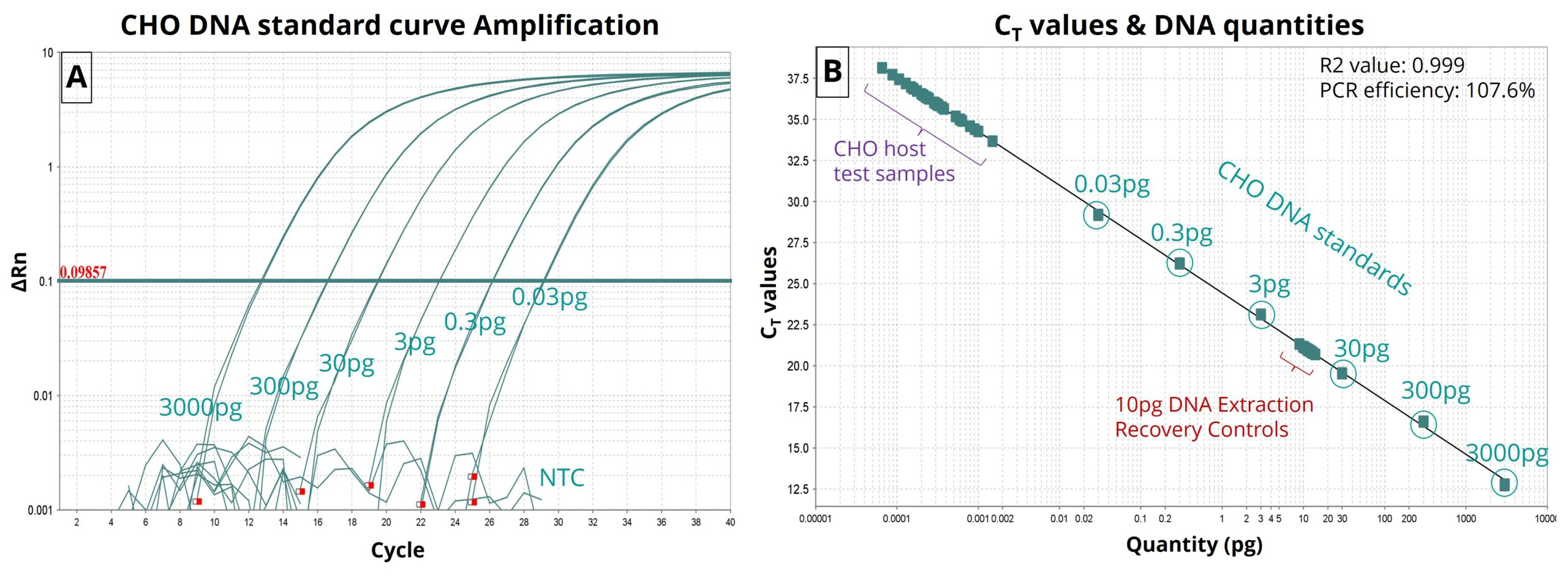
Figure-1: (A) CHO DNA standard curve amplification along with NTC. (B) CT value-vs-DNA quantity (pg) plot of standards along with residual CHO DNA in Therapeutic protein test samples and Extraction Recovery control samples. Therapeutic protein test samples exhibited femtogram levels of CHO residual DNA below the lowest concentration of CHO DNA standard. Assay exhibited 107.6% PCR efficiency with an R2 value of 0.999. Recovery controls (10pg) performed well within range.
Residual E.coli cell line DNA Quantification
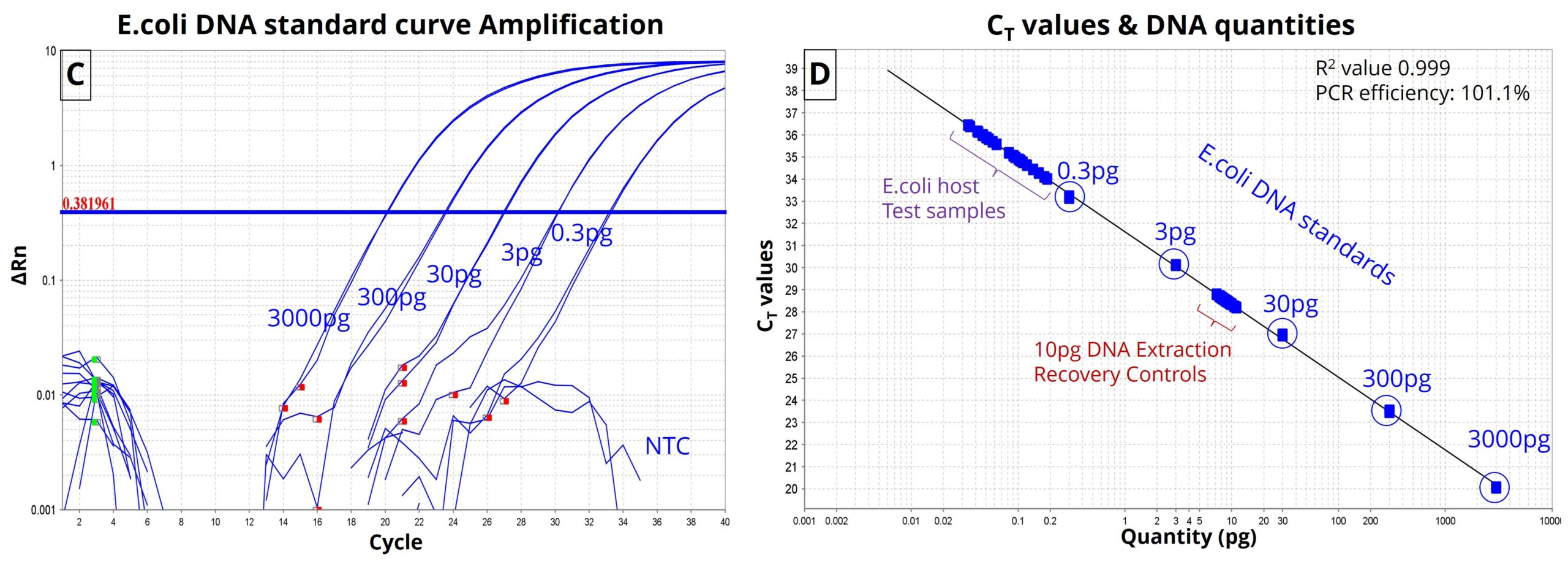
Figure-2: (C) E.coli DNA standard curve amplification along with NTC. (D) CT value-vs-DNA quantity (pg) plot of standards along with residual E.coli DNA in Therapeutic protein test samples and Extraction Recovery control samples. Therapeutic protein test samples exhibited sub-picogram levels of E.coli residual DNA below the lowest concentration of E.coli DNA standard. Assay exhibited 101.1% PCR efficiency with an R2 value of 0.999. Recovery controls performed well within range.
Therapeutic protein test samples from CHO host and E.coli host exhibited residual host DNA levels within acceptable limits. Extraction recovery samples exhibited good extraction recovery within the acceptance criteria. Residual host cell DNA quantification assays developed at BioMedha are robust tools for oncogenicity and/or immunogenicity risk mitigation in non-GLP format.